Sorbic Acid — toxicity, side effects, diseases and environmental impacts
Tuesday, November 14, 2017 by Michelle Simmons
http://www.naturalpedia.com/sorbic-acid-toxicity-side-effects-diseases-and-environmental-impacts.html
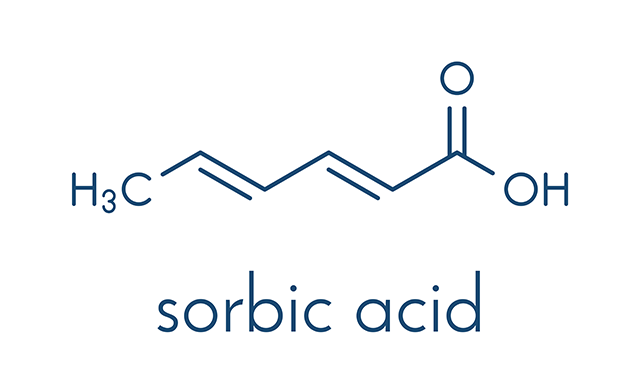
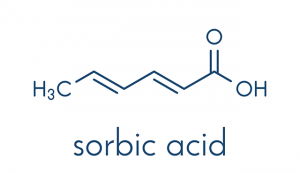
Sorbic acid is a type of acid that can be obtained from the fruit of the European Mountain Ash (Sorbus aucuparia), after which it was named. Although this acid naturally occurs, it is commercially manufactured through several different chemical pathways from the toxic, irritant, colorless gas known as ketene.
Sorbic acid is used as a food preservative because it prevents spoilage caused by yeasts, fungis, and molds, but allows the activity of bacteria. This unsaturated fatty acid is also used mainly in the production of facial and eye makeup, skin care, and hair products.
According to an entry by BeFoodSmart.com, sorbic acid can also be identified in these names: calcium sorbate, potassium sorbate, sodium sorbate, acetic acid, hexadienic acid, hexadienoic acid, and sorbistat. Sorbic acid has the molecular formula of C6H8O2 and has an E number of E200.
List of known side effects
There is not much information on the possible side effects of sorbic acid on humans. One of the few side effects of sorbic acid is that it can cause irritation on the skin on some people, particularly those who are allergic to it. Its symptoms, however, are typically mild and consist of light itching on the skin. Although rare, allergic contact may result to dermatitis. This acid should also be avoided by people with eczema due to possible irritation. There is no information on its harmful effect in the environment.
According to the Centers of Disease Control and Prevention (CDC), inhalation of the acid can cause sore throat and cough, while contact in the eyes can cause redness, pain, and blurred vision. Ingestion of sorbic acid can also lead to a burning sensation.
Body systems affected by sorbic acid
There are a few body systems that can be affected by sorbic acid when handling it in its pure form. One of these is the integumentary system as it may cause irritation on the skin. Another system that can be affected by this acid is the ocular system because it can cause serious eye irritation or damage when in contact. Sorbic acid can also adversely affect the respiratory system as it may cause respiratory tract irritation if inhaled.
Items that can contain sorbic acid
According to the list posted by LiveStrong.com, sorbic acid can be found in food products, especially packaged or processed foods. Other food products that may contain sorbic acid are dairy foods such as cheese and yogurt, candied peel, cider, dessert sauces, fillings and toppings, fermented milks, frozen pizzas, fruit salads, gelatin capsules, soft drinks, soup concentrates, sweets, dried fruit, fish, meat, pickles, olives, soups, prepared salads, jelly, syrups, wine, beer, soft drinks, and baked products such as breads, bagels, and pastries. Sorbic acid can also be found in non-food products such as animal feeds, pharmaceutical drugs, and cosmetics.
How to avoid sorbic acid
According to the CDC, sorbic acid can be avoided in several ways. One of these ways is to have a local exhaust or use a breathing protection. Using protective gloves and clothing is also a way to prevent skin contamination with sorbic acid. Another way is to use safety spectacles or eye protection in combination with breathing protection to avoid the substance from getting in contact with your eyes. To avoid ingestion of the acid, simply avoid eating, drinking, or using products that may contain sorbic acid.
Where to learn more
- Kraft removes sorbic acid preservative from some ‘Singles’ products, replaces it with GMOs and an unnamed, proprietary stabilizer
- Are You Being Poisoned On a Daily Basis?
- Our Favorite Junk Foods are Changing
- Kraft Macaroni and Cheese Recalled Due to Metal Fragments
- Titanium dioxide in vitamins and supplements: Is it safe for human consumption?
Summary
Sorbic acid is a type of acid that is used as a preservative as it prevents spoilage caused by yeasts, fungis, and molds. Although it naturally occurs, it is commercially manufactured chemically from the toxic, irritant, colorless gas known as ketene.
Sorbic acid can cause irritations, sore throat, cough, redness and pain in the eyes, blurred vision, and a burning sensation in the gastrointestinal tract.
Sorbic acid is harmful for the integumentary, ocular, and respiratory systems.
Sources include:
Tagged Under: Tags: Sorbic acid